SCIENCE OF PLASTICS
Plastics (polymers made from organic chemicals) have such useful properties that they are found in all sorts of products from tyres and textiles to toothpaste and tupperware. As these break down they produce smaller and smaller particles - microplastics and their even smaller counterparts nanoplastics. These particles can then travel all over the world simply by being blown in the wind. Their unusual properties mean that it may not be a good idea for us to inhale them or let them become part of the food chain.
What Is Plastic?
Plastics are hydrocarbon-based polymers (compounds made up of repeating strings of smaller molecules known as monomers) usually made from crude oil or natural gas. Unlike other polymers such as rubber, latex, cellulose, wool, DNA or proteins, plastics are malleable and can be moulded into solid objects which retain their shape.
The type of plastic depends on the monomer from which it is made. Some monomers form linear chains, others a simple branch structure and in others the long monomer chains become linked to each other and are referred to as networked or cross-linked polymers. The arrangement of the monomers and which additional elements are attached to the central backbone determine the physical properties of any given plastic. Most are relatively inert, have low electrical and thermal conductivity and a high strength:weight ratio. Some melt when heated and can be melted and reshaped; others remain set after curing and do not melt.
How Many Different Sorts of Plastic Are There?
Industrially, only half a dozen types of plastic are in common use - polyethylene (PE) (high and low density), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), polyurethane (PUR) and polyethylene terephthalate (PET). Note that polyethylene comes in two types - high and low density. Low density polyethylene is ubiquitous in the form of plastic bags. High density polyethylene, on the other hand, is very inert and often used to make containers for motor oil, antifreeze, household cleaning products etc..
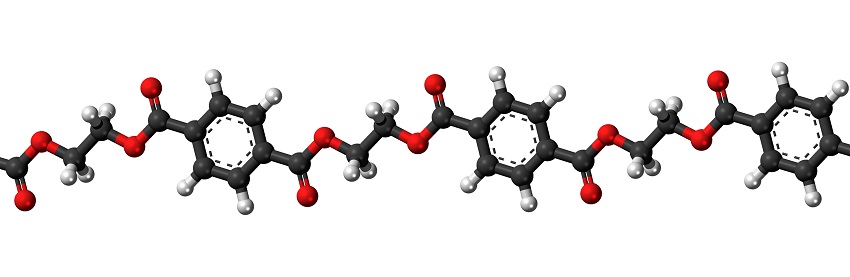 The diagram above shows the structure of the commone plastic polyethylene terephthalate (PET). You can see that the monomer is a ring of six carbon atoms (shown in black), with their attached hydrogen atoms (in white) and pairs of oxygen atoms at either side. As it is cheaper and less fragile than glass, PET is most commonly used for plastic bottles.
The diagram above shows the structure of the commone plastic polyethylene terephthalate (PET). You can see that the monomer is a ring of six carbon atoms (shown in black), with their attached hydrogen atoms (in white) and pairs of oxygen atoms at either side. As it is cheaper and less fragile than glass, PET is most commonly used for plastic bottles.
What Is Plastic Used For?
Industrially, there are only half a dozen types of plastic in common use - polyethylene (PE) (high and low density), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), polyurethane (PUR) and polyethylene terephthalate (PET). Note that polyethylene comes in two types - high and low density. Low density polyethylene is ubiquitous in the form of plastic bags. High density polyethylene, on the other hand, is very inert and is often used to make containers for motor oil, antifreeze, household cleaning products etc..
| Type | Abbreviation | Structure | Major Uses | Market Size $USbn (2022) | Market Size (millions of tons) |
| Polyethylene | PE | 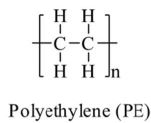 |
Bags, packaging, tupperware and plastic pipes | $110bn | 53 |
| Polypropylene | PP | 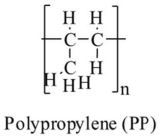 |
Textiles, packaging, automotive industry | $130bn | 74 |
| Polyvinyl Chloride | PVC | 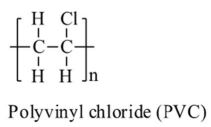 |
Construction (pipes, windows, cabling), medical tubing and bags, waterproof goods | $70bn | 45 |
| Polytsyrene | PS | 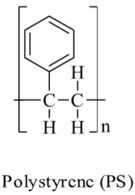 |
Insulation, disposable cutlery, plates and food containers, styrofoam and expanded polystyrene packaging. | $30bn | 11 |
| Polyurethane | PU | 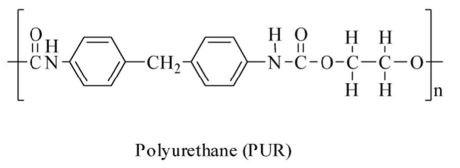 |
Insulation, flexible foam, coatings and sealants. High use in the automotive industry. | $80bn | 25 |
| Polyethylene Terephthalate | PET | 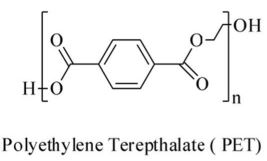 |
Plastic drinks bottles, food packaging and blister packs, textiles. | $45bn | 30 |
Further information on the various types of plastic is available from industry news source NS Packaging.
What Are Nano- and Microplastics?
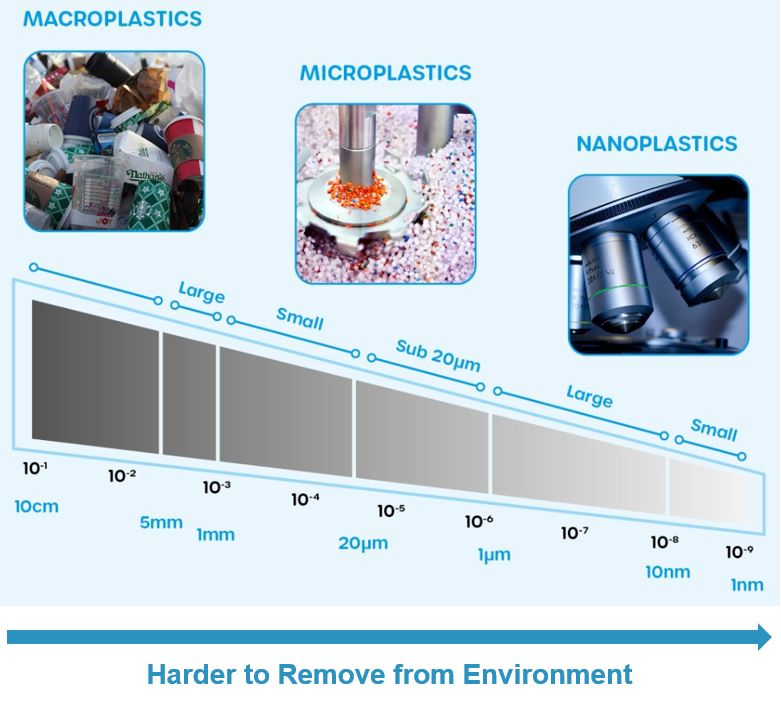
Plastic pollution is generally described in terms of its size. The plastic bags found floating amongst the 'great pacific garbage patch' are generically referred to as macroplastics. There have been various definitions of smaller plastic waste but, generally, microplastics are particles of plastic less than 5mm in size. Their even smaller counterparts, nanoplastics, are particles less than 1μm (a millionth of a metre) in size.
Macroplastics - old plastic bags and empty bottles - are easy to see. Microplastics are small enough that it can be hard to see them but the early measurements of microplastics on glaciers were done using nothing more sophisticated than microscopes. With very small nanoplastics, however, even the best optical microscopes won't do. Besides, counting microplastic particles by hand isn't very reliable and doesn't tell you what sort of plastic they are. You need to use other methods such as spectroscopy.
Macroplastics - old plastic bags and empty bottles - are easy to see. Microplastics are small enough that it can be hard to see them but the early measurements of microplastics on glaciers were done using nothing more sophisticated than microscopes. With very small nanoplastics, however, even the best optical microscopes won't do. Besides, counting microplastic particles by hand isn't very reliable and doesn't tell you what sort of plastic they are. You need to use other methods such as spectroscopy.
The Plastic Cycle & The Origin Of Microplastics
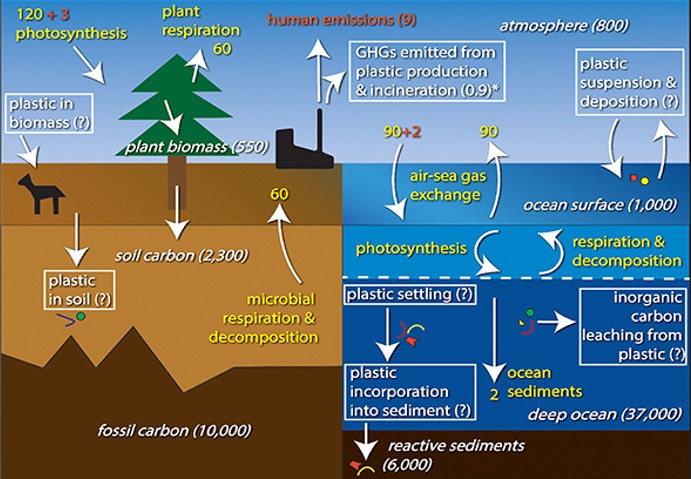 You probably remember the carbon cycle from school. Animals breathe in oxygen and breathe out carbon dioxide. Plants, algae and some bacteria then breathe in carbon dioxide which they turn into organic compounds like glucose during photosynthesis and produce oxygen in the process. Other factors affect this cycle:-
You probably remember the carbon cycle from school. Animals breathe in oxygen and breathe out carbon dioxide. Plants, algae and some bacteria then breathe in carbon dioxide which they turn into organic compounds like glucose during photosynthesis and produce oxygen in the process. Other factors affect this cycle:-
- dead animals and plants decay and return carbon to the soil
- under pressure, dead organic matter eventually produces coal, oil or natural gas
- the world's oceans absorb carbon from the atmosphere
- combustion of wood, coal and petrochemicals releases carbon dioxide into the atmosphere
- erosion of rocks releases carbon and carbonates
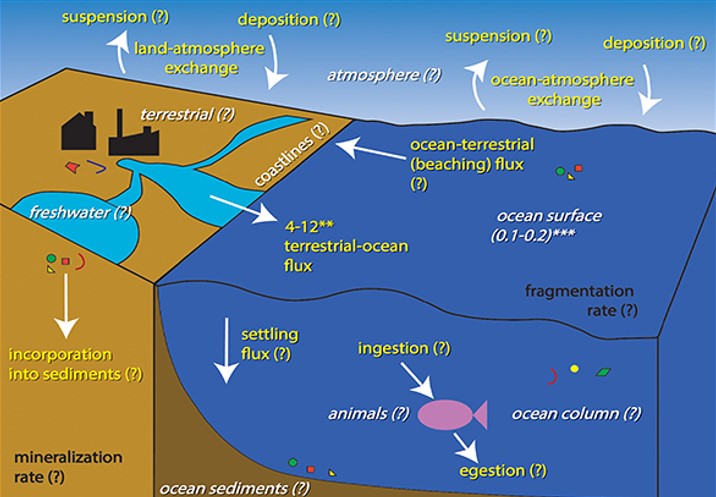 The plastic cycle is similar. It is also linked to the carbon cycle via the petrochemicals which are the feedstock for the manufacture of plastics.
The plastic cycle is similar. It is also linked to the carbon cycle via the petrochemicals which are the feedstock for the manufacture of plastics.
These plastics are then turned into various commercial products which are used and, ultimately, thrown away. Either very quickly in the case of single use polystyrene cutlery, or after a decade or so in the case of automotive plastics.
Sometimes, particularly with PET, the plastic is recycled but, more often than not, the plastic makes its way to landfill, rivers or oceans or is just dumped. It then starts to decompose, but this takes longer than for organic waste. Depending on the type of plastic and the environment, this can take anything from 10-500 years.
This breakdown of plastics takes place in one of three ways:-
- mechanically - by wind or wave action or by being cut up for recycling
- chemically - exposure to UV or heat slowly oxidizes plastics and makes them prone to fragmentation
- biologically - certain bacteria have evolved to eat plastic
Blown in the wind, it is these microplastics and nanoplastics which end up deposited in the snow which lies on the surfaces of glaciers and ice caps. This snow then serves as a natural repository - a frozen snapshot of the microplastics blowing around in the atmosphere - and holds onto them until we come along to sample them.
A more detailed discussion of the plastic cycle can be found here.
How Do We Measure Them?
The advantage of our measurement technique is that it lets us measure not only microplastics but also their smaller nanoplastic counterparts. This is difficult, if not impossible, with most conventional means.

Prototype TD-PTR-MS Equipment
The photograph above shows a prototype of the system developed by Dr Materić to measure microplastics at the Sonnblick Observatory in Austria. This is a precursor of the equipment used for the HLR 2022 Expedition and the more advanced system at the laboratory in Leipzig which will be used for GAPS 2024-25.
The measurement process is called Thermal Desorption Proton Transfer Reaction Mass Spectroscopy (TD-PTR-MS). It works as follows:-
- we evaporate the water from the samples in a clean environment to prevent contamination.
- this leaves any solid volatile organic compounds (VOCs) such as plastics on a substrate
- controlled heating turns any nano- or microplastics into a gas (thermal desorption)
- we use a radioactive source to ionise water and produce with H3O+ ions
- these H3O+ ions are combined with the VOC vapour in a separate reaction chamber.
- ionised hydrogen atoms (protons) transfer across to the gaseous plastics making them charged (proton transfer reaction)
- these charged gaseous plastics are then introduced into a mass spectrometer and measured in the usual way. (mass spectroscopy)
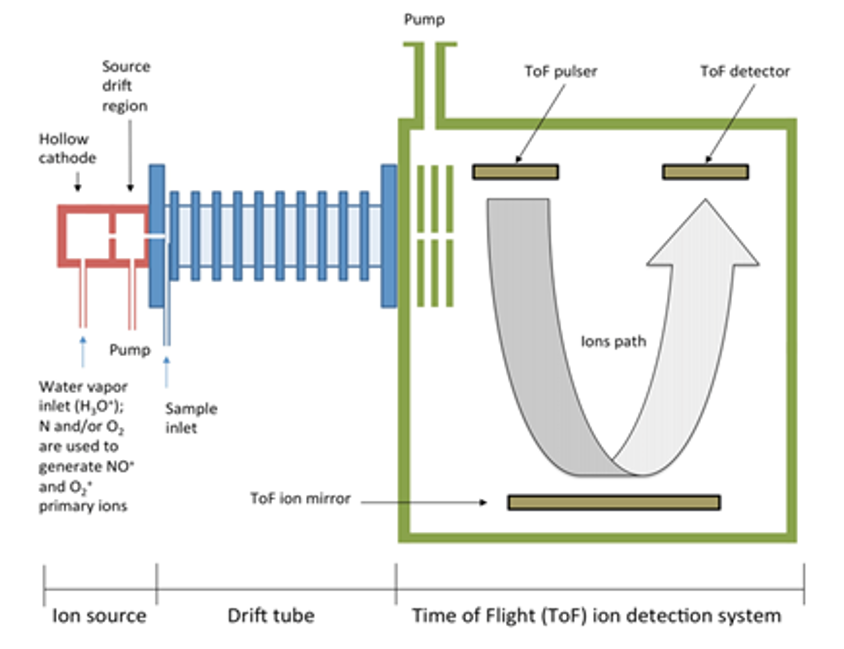 TD-PTR-MS is more sensitive than other techniques and can be adapted to measure the size distribution of the microplastics. On the flip side, it does have severalsteps and requires skilled laboratory staff. The mass spectroscopy part of the process, however, is entirely standard.
TD-PTR-MS is more sensitive than other techniques and can be adapted to measure the size distribution of the microplastics. On the flip side, it does have severalsteps and requires skilled laboratory staff. The mass spectroscopy part of the process, however, is entirely standard.
- the ionised lengths of plastic polymer are accelerated using an electric field
- they are then passed through a magnetic field
- the lighter the ionised polymer lengths are, the more they are deflected by the magnetic field.
- just like splitting light with a prism this results in a spectrum of ionised polymers arranged by mass
- calculating the masses of the various types of plastic monomer allows us to identify the various plastics
What Will This Tell Us?
Conducting a global survey, measuring the plastics using TD-PT-RMS and combining this with atmospheric modelling we hope to find out:-
- what types of plastic are getting into the atmosphere?
- the size distribution of the various types of airborne microplastic
- where the plastics come from and what routes they take
Where Can I Find Out More?
Plastics Europe - Industry Body
Ocean Care - Not-for-Profit Consulting and Lobbying Group
Wasser Drei Null - Not-for-Profit Research and Education Organisation
NS Packaging - Industry New Source.
Ocean Care - Not-for-Profit Consulting and Lobbying Group
Wasser Drei Null - Not-for-Profit Research and Education Organisation
NS Packaging - Industry New Source.




